FOCU·SE is a new Swedish study in which genetic analysis of tumours is used to match cancer patients with targeted drugs outside of approved indication. The aim is to give more people access to effective treatment, while gathering valuable knowledge for the future.
An initiative by Testbed Sweden Precision Health Cancer, founded by Vision Zero Cancer, Genomic Medicine Sweden and SciLifeLab, involving regulators, policymakers, payers, researchers, healthcare providers, patient advocates, and industry to drive the implementation of precision health in cancer care.
FOCU·SE – National study for equitable implementation of precision medicine in cancer care
Background and purpose
FOCU·SE is a national clinical and exploratory precision medicine study for advanced cancer. The study has been developed within the project Testbed Sweden Precision Health Cancer, with funding from Vinnova and Swelife, in broad collaboration between Genomic Medicine Sweden (GMS), SciLifeLab, university hospitals, industry partners and patient organizations. The aim is to enable individualized treatment for patients with advanced cancer through biomarker-guided treatment outside of established indication. FOCU·SE is linked to the European initiatives PCM4EU and PRIME-ROSE.
Overall objectives and study design
FOCU·SE aims to, through broad molecular profiling and national coordination via the Molecular Tumor Boards (MTB), increase access to targeted cancer drugs for patients with advanced cancer who lack treatment options. The study enables systematic testing of EMA-approved drugs outside of indication (drug repurposing), which speeds up the path to approved new treatments while monitoring the clinical benefit and safety in real time.
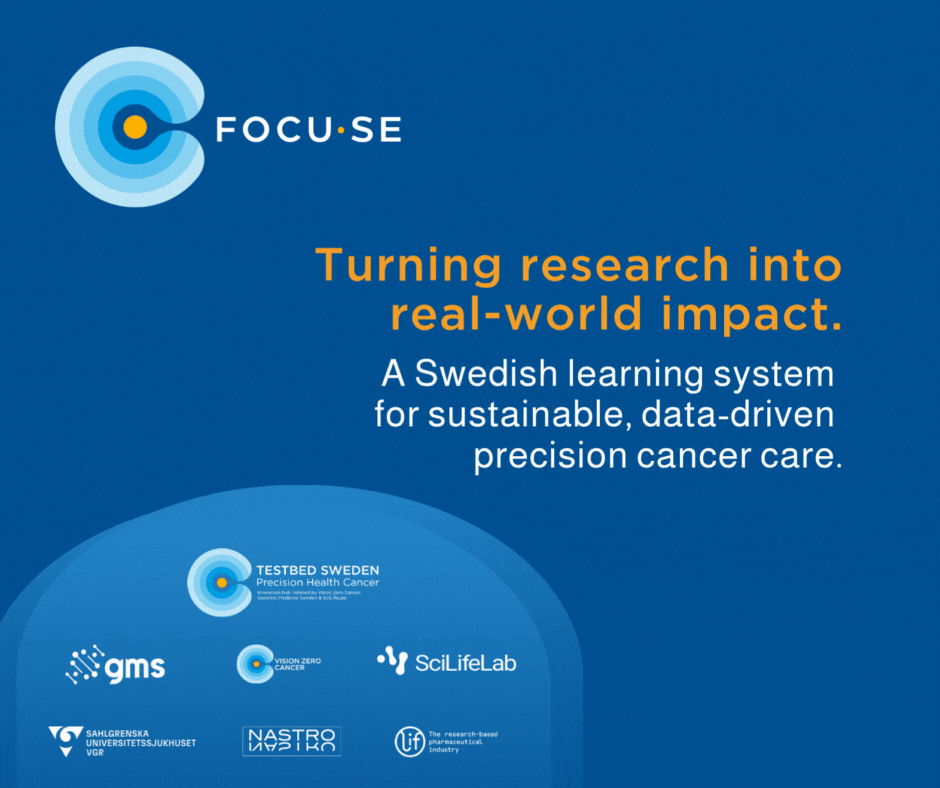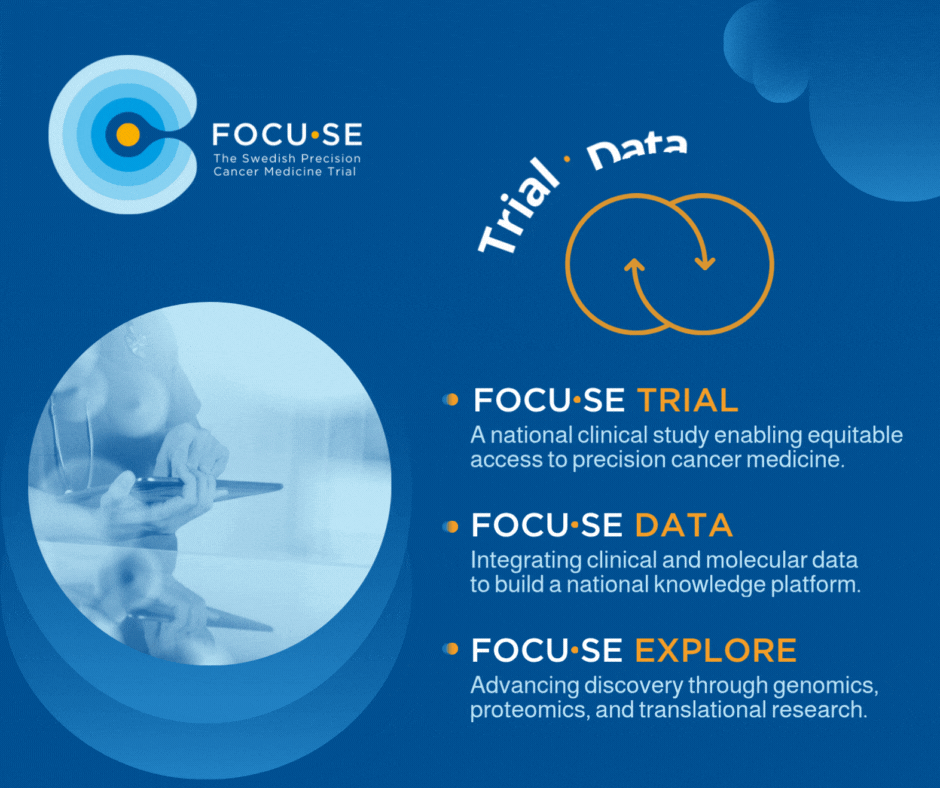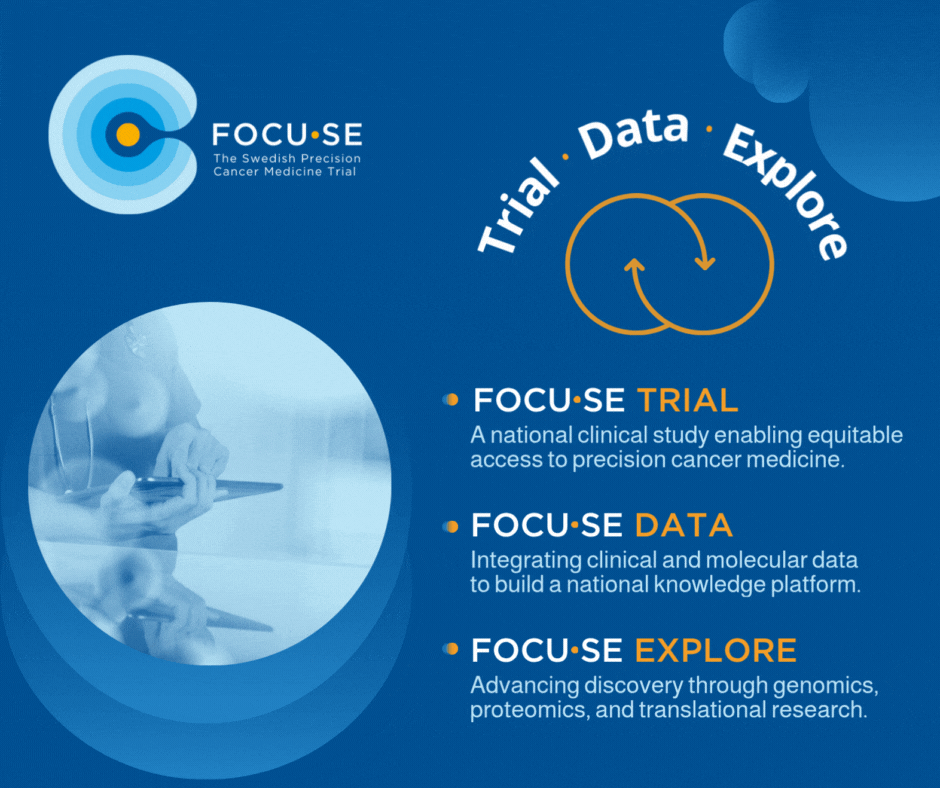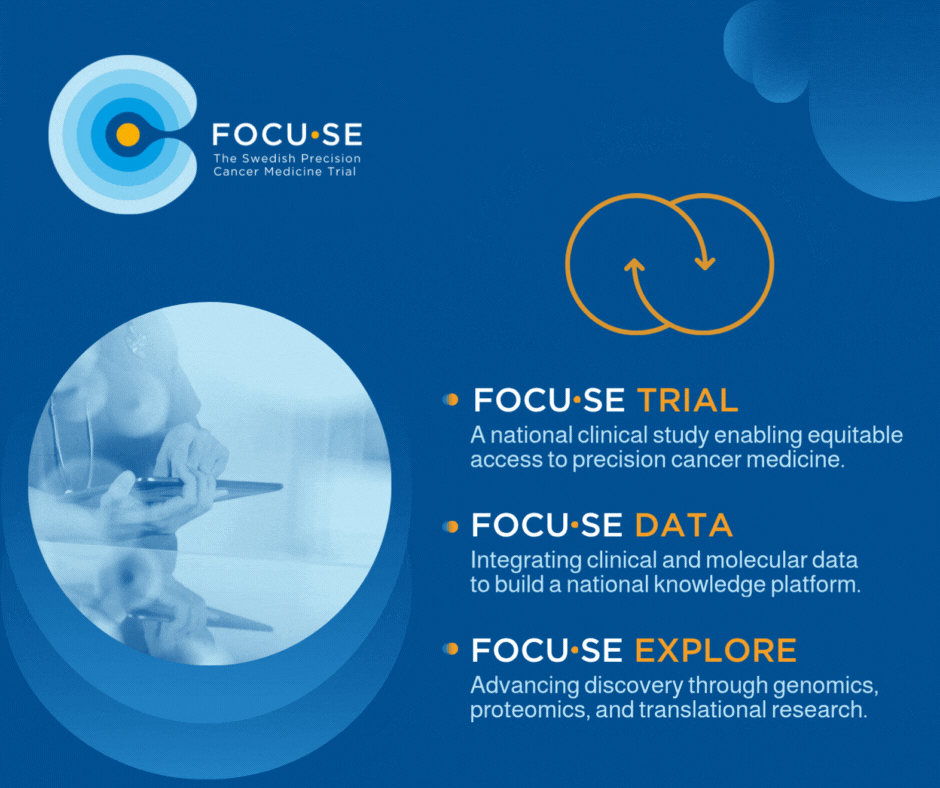
The study, FOCU·SE -trial, is structured as a platform study with cohorts based on matching between biomarkers and available drugs. The cohorts are evaluated according to the Simon 2-step model and include 8–16 patients depending on treatment response. Up to 1000 patients are screened annually to include approximately 250 patients per year. All patients undergo genetic profiling via GMS560 panel or other validated method, including ctDNA. All results are presented in a national MTB. The PI for the study is Edvard Abel, MD, PhD, Sahlgrenska University Hospital.
The exploration part, FOCU·SE -explore, uses techniques such as whole genome sequencing and proteomics to understand treatment resistance and identify new biomarkers. A data integration component (FOCU·SE data) connects clinical and molecular data via an eCRF and builds up a national knowledge bank for systematic learning.
Figure 1. FOCU·SE. In addition to the clinical study, it includes an exploratory part and a part focused on data integration. WGS, whole- genome sequencing, WTS, whole-transcriptome sequencing, IHC, immunohistochemistry. Medicines in FOCU·SE
FOCU·SE use EMA-approved targeted cancer drugs that are tested outside their current approved indications, according to the DRUP model. This enables so-called Drug Repurposing, where already available drugs are used for new patient groups based on biomarker profiling.
The model also provides regulatory and scientific data for future indications, which can accelerate approval processes and streamline drug use in healthcare. In addition, it gives patients with limited treatment options access to promising therapies, while reducing the side effects and costs of ineffective treatment.
The value of structured follow-up of off-label treatments
Some of the drugs currently used in precision medicine are already given off-label to individual patients when treating clinicians see a potential benefit, based on biomarker data. However, these treatments often take place without systematic follow-up or structured data collection.
FOCU·SE offers a unique opportunity to collect, analyze and share outcome data from off-label treatments in a standardized and quality-assured way. This provides several added values:
• This enables evidence building up about effects and side effects in new patient groups.
• Documentation can be generated for future approvals and recommendations.
• Treatment decisions can be improved over time through learning in national MTB.
This systematic follow-up strengthens patient safety, reduces uncertainty in care decisions and contributes to a more cost-effective use of drugs in cancer care.
FOCU·SE as a platform for future treatments
FOCU·SE is not only a clinical trial but also serves as a national platform for screening and testing patients for inclusion in precision medicine treatment studies. Through the built-up infrastructure for molecular testing and national HTA process, patients can be quickly identified for participation in new clinical trials, even outside of the FOCU itself. SE Protocol.
This capacity positions Sweden as an attractive country for drug trials in the cancer area and strengthens Sweden's competitiveness in life science. The platform's flexibility to include new treatments and biomarkers creates good conditions for innovation and international collaboration.
FOCU·SE can thus serve as a hub for future drug trials, including combination treatments, in collaboration with industry.
Infrastructure and strategic proposals
To ensure equuitable implementation, it is proposed:
That 500–1500 broad gene panels are reserved annually within the framework of the cancer strategy's five-year biomarker initiative.
Establishment of a national structure for the Molecular Tumor Board Portal (MTBP).
Coordinated investment in data infrastructure for clinical studies, decision support and integrated follow-up.
Return on investment (ROI)
1. Health economic benefits
Reduced overtreatment and side effects through targeted treatment.
Prolonged survival and increased quality of life (average +6 months).
More efficient use of medicines and treatment choices.
2. System Efficiency
Faster regulatory decisions on expanded indications.
Efficient data sharing and clinical decision support via national MTB.
Reducing regional disparities through national coordination.
3. Industrial and scientific leverage
Sweden as a pioneer country in PCM, attractive for international studies.
The industry's participation in the study provides long-term clinical benefit.
Access to EU funding through, among others, PRIME-ROSE, EU4Health, Horizon Europe.
FOCU·SE is a key in the implementation of sustainable, efficient and equitable precision cancer care in Sweden. State support is crucial to ensure national coverage and future patient benefit.
The Swedish Precision Cancer Medicine Trial FOCU·SE
Enhance advanced cancer care in Sweden with a nationwide Precision Cancer Medicine trial.
The upcoming trial, inspired by the Drug Rediscovery Protocol (DRUP) family of trials, utilizes a prospective, non-randomized Simon two-stage design. Patients are selected based on thorough molecular profiling and closely monitored for residual disease.
Building on Swedish expertise in multi-omics and drug sensitivity screening, we aim to discover novel predictive biomarkers and therapeutic targets, leading the way for the next generation of precision medicine and improving clinical outcomes
Figure: Clinical Trial Patient Journey
Expected outcomes
A national clinical study platform contributing to DRUP-like trials.
Increased opportunities for rare cancer patients to test promising therapies.
Implementation of a national Molecular Tumour Board (MTB).
A biomarker research engine for basic research and innovative clinical trials.
Elevating Sweden as a clinical trial leader in precision cancer medicine.
Drug rediscovery
Drug rediscovery studies have proved an effective means to help individual patients to novel treatments and add new indications to existing targeted therapies. The application of precision medicine in clinical trials also helps refine processes and establish infrastructure for diagnosis, treatment, and follow-up, contributing to the successful implementation of precision medicine.
Shared DRUP core trial design
Simon two-stage model
Eligible patients with identified actionable targets with matching drug from the study portfolio will be included in a cohort.
A cohort will consist of patients with same indication and the same actionable target.
Multi-step evaluation of response.
Latest news about FOCU.SE >>>
MULTI-STAKEHOLDER COLLABORATION
Precision cancer medicine requires expertise from various fields. FOCU·SE is an initiative by Testbed Sweden Precision Health Cancer, founded by Vision Zero Cancer, Genomic Medicine Sweden and SciLifeLab, involving regulators, policymakers, payers, researchers, healthcare providers, patient advocates, and industry to drive the implementation of precision health in cancer care.
STAKEHOLDER VALUE
Society: Equitable access to precision cancer medicine.
Patients: Added diagnostic and treatment options.
Clinicians: Expanded treatment choices.
Researchers: Possibility for basic research and translation of results into clinical practice.
Pharma: Introduction of new precision medicine indications.
Medtech: Validation of diagnostics and devices.
Policy makers: Opportunity to learn from an implementation model.
HTA: Real-world cost-effectiveness.
Payors: Access to precision cancer medicine for patients.
DLCT COMMUNITY
FOCUSE is part of a family of independent, investigator-initiated, pragmatic clinical trials in precision medicine inspired by the design of the original DRUP protocol.